IIT JAM Biology 2021
Previous Year Question Paper with Solution.
1. Ethyl butyrate is responsible for the odor of pineapple. Which one of the following is the structure of ethyl butyrate?
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
Option (d) is correct.
2. 
(a) –6
(b) 6
(c) –18
(d) 18
Ans. (d)
Sol.


3. If the blood groups of mother and father are AB and O, respectively, what are the blood groups possible for their child?
(a) AB or A
(b) AB
(c) AB, A, B or O
(d) A or B
Ans. (d)
Sol. Mother's blood group is AB, so antigen on mother's RBC Would be IA and IB father's blood group is O, so antigen on father's RBC would be iO and iO. So, possible blood group of the child could be

IiA is 'A' blood group and IiB is 'B' blood group.
So, option (d) is correct.
4. Which one of the following components of bacterial cell acts as endotoxin?
(a) Porins
(b) Lipopolysaccharide
(c) Peptidoglycan of Gram-positive bacteria
(d) Peptidoglycan of Gram-negative bacteria
Ans. (b)
Sol. Endotoxins are the component of the outer membrane of bacterial wall. Endotoxins refer to the lipopolysaccharide complex associated with the outer membrane of Gram negative bacteria.
So, correct option is (b)
5. The type of immunological protection provided by plasma therapy is
(a) natural active
(b) artificial passive
(c) natural passive
(d) artificial active
Ans. (b)
Sol. Plasma therapy is a type of passive immunisation process. In this therapy transfusion of plasma containing antibodies from immune survivors of infectious disease to the affected individual is done. Passive immunisation can be either natural or acquired. When an infant receives antibodies from mother's milk, it is natural passive but in plasma therapy as this transfer happens artificially, So option (b) is correct.
6. An acid contains C, H and O atoms. On combustion analysis, 0.454 g of the acid gives 0.418g of H2O and 1.023 g of CO2. What is the empirical formula of the acid?
(a) C4H5O2
(b) C5H8O
(c) CH2O
(d) C3H6O
Ans. (d)
Sol. Atomic mass of H = 1.0079
Atomic mass of C = 12.0107
Atomic mass of O = 16 amu

Amount of H and C obtained from combustion are
Hydrogen = 0.1119 × 0.418 = 0.046
Carbon = 0.02727 × 1.023 = 0.2727
Mass of oxygen
= 0.450 – 0.046 – 0.2727 O2 = 0.124 cm
Molecular mass of caproic acid = 116.2

7. The moment of force in terms of fundamental dimensions is
(a) ML–1T–1
(b) MLT–2
(c) MLT–1
(d) ML2T–21
Ans. (d)
Sol. Moment of force = force × distance
Dimenstional fourmula of force = [MLT–2]
Dimenstional formula of distance = [L]
So, dimensional formula of moment of force is = [MLT–2] [L] = [ML2T–2]
Hence, option (d) is correct.
8. Ecosystem ecology is the study of
(a) An organism's behavior towards environmental challenges
(b) Factors that affect the interactions among communities in an ecosystem
(c) Interactions among biotic and abiotic components
(d) Factors that affect the interactions of individuals in a population
Ans. (c)
Sol. Ecosystem ecology is the combined study of living (biotic) and non-living (abiotic) component of an ecosystem and their interactions. So option (c) is correct.
9. Bacterial strains that do not grow in the absence of a specific nutrients are called
(a) autotrophs
(b) heterotrophs
(c) auxotrophs
(d) chemotrophs
Ans. (c)
Sol. Auxotrophs are mutant bacterial strains that required a particular additional nutrient which the normal strain does not require. If specific nutrient is not given then auxotroph will not grow.
So, option (c) is correct.
10. Which one of the following features distinguishes between gymnosperms and angiosperms?
(a) Vascular tissues
(b) Seed cover
(c) Seed formation
(d) Gamete production
Ans. (b)
Sol. Angiosperms also referred to as flowering plants have seeds that are enclosed in an ovary, which later turns into a seed that are enclosed in an ovary, which later turns into a seed coat, whereas gymnosperms are non-flowering plants and have naked seeds which are not enclosed within a seed coat. So, option (b) is correct.
11. The difference between mitosis and meiosis I is
(a) The DNA is double helical in meiosis I, but not in mitosis
(b) The nuclear membrane is absent during mitotic metaphase, but not in meiotic metaphase
(c) Sister chromatids separate in mitosis, whereas homologous chromosomes separate in meiosis I
(d) Unlike in mitotic metaphase, chromosomes do not align at the equatorial plate in meiosis I
Ans. (c)
Sol. Mitosis is a process of cell division, in which a cell divide to produce two genetically identical daughter cells. Mitosis consists of various stages in which cells's chromosome are copied and then distributed equally between two nuclei of daughter cells.
Meiosis on the other hand is used for the production of gametes only. It also reduces chromosome number to half in gametes. Meiosis occurs in two stages meiosis I and meiosis II. In meisosi I homogous chromosome separate whereas in meiosis II sister chromatids separate. Mitosis includes separation of sister chromatids only. So, option (c) is correct.
12. Which one of the following features/properties does glucose acquire through intramolecular hemiacetal formation?
(a) Ability to form epimers
(b) Ability of function as a reducing agent
(c) An additional chiral carbon
(d) Ability to form anhydride linkage with non-carbohydrate moieties such as the inorganic phosphate
Ans. (c)
Sol. The cyclic form of glucose is a six-membered ring, An intramolecular hemiacetal formed by attack of the hydroxyl on the fifth carbon on the aldehyde carbon (C1). This carbon is called the anomeric carbon in carbohydrate terminology.

13. Which one of the following microscopic techniques provide a 3-dimensional perspective of live unstained and transparent specimens obtained from the wild?
(a) Fluorescence microscopy
(b) Differential interference contrast (Nomarski) microscopy
(c) Confocal microscopy
(d) Phase contrast microscopy
Ans. (b)
Sol. Differential Interference Contrast (DIC) microscopy, also known as Nomarski microscopy is a technique used to enhance contrast in unstained, transparent sample. It consists of complex optical system which produces image of a sample even without staining. So, option (b) is correct.
14.

(a) – 2
(b) – 1
(c) 2
(d) 1
Ans. (c)
Sol.


15. Match the cell junctions listed in Group-A with their correct functions listed in Group-B.

(a) I-P; II-Q; III-R; IV-S
(b) I-Q; II-R; III-P; IV-S
(c) I-S; II-P; III-Q; IV-R
(d) I-Q; II-R; III-S; IV-P
Ans. (a)
Sol. (i) Adherens junction are a type of problem complexes that help in cell-cell adhesion. The cytoplasmic face of adherens junction is linked to actin cytokeleton.
(ii) Desmosomes are also adhesive protein complexes but they are localised to intercellular junctions and are responsible for maintaining mechanical integrity of tissues.
(iii) Tight junction are selectively permeable seals in our body's internal and external sufaces.
(iv) Gap junction are membrane channels that allows cell to cell movement of ions and small metabolities. So, option (a) is correct.
16. Which one of the following is the major product of the hydrogenation reaction given below?
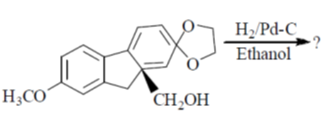
(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol.

We cannot add 'H' in the first ring, because it will destroy aromaticity, so during hydrogenation the addition of hydrogen will take place to the other double bond which will maintain stability and reduce steric hinderence.
Thus, option (c) is correct.
17. The following methylation is carried out in various solvents such as benzene, tetrahydrofuran (THF), dimethoxyethane (DME), dimethyl sulfoxide (DMSO) and N, N-dimethyloformamide (DMF). Which one of the following is true for the effect of solvent on the reaction rate?

(a) DME > DMSO > DMF > THF > Benzene
(b) Benzene > THF > DME > DMF > DMSO
(c) THF > Benzene > DME > DMSO > DMF
(d) DMSO > DMF > DME > THF > Benzene
Ans. (d)
Sol. There are several factors that effect that effect the rate of solvation. These include, temperature, concentration, surface area of solute, concentration of solvate and stirring. The general reason for an increase in rate of solvation is that solute molecules come into contact with solvent molecules more often. So, DMSO solvates cation more easier as compared to te anion and will have the highest reaction rate. Thus, option (d) is correct.
18. In mammals, females have two X xhromosomes and males have one X chromosome. Equal expression of X-chromosome genes in both sexes is ensured by
(a) RNA silencing
(b) Histone code
(c) Dosage compensation
(d) Heterochromatin formation
Ans. (c)
Sol. Dosage compensation is the process by which organism equalise the expression of genes and sex chromosome in mammals and organisms of other species. Hence, option (c) is correct.
19. Which one of the following represents the motion of an object with a positive acceleration?
(a)

(b)

(c)

(d)
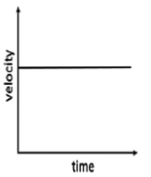
Ans. (a)
Sol. For positive acceleration, the slope of tangent drawn at each point on velocity time graph should be positive.
Among all given graph only (a) is correct representation for graph (c) velocity is uniform and that for graph (d) is zero, so for both graph acceleration is zero.
20. What is the significance of the isomerization of glucose 6-phosphate to fructose 6-phosphate for the progression of glycolysis?
(a) Phosphorylation of glucose 6-phosphate to glucose 1, 6-bisphosphate is irreversible
(b) The carbonyl group at carbon-2 (C-2) in fructose facilitates the cleavage of the bond between C-3 and C-4.
(c) Cleavage of glucose 1, 6-biosphosphate will not yield dihydroxy acetone phosphate and glyceraldehyde 3-phosphate
(d) As functional groups, ketones are more reactive than aldehydes
Ans. (b)
Sol. Glycolysis is the process of breakdown of glucose in a stepwise manner. Glucose is firstly converted into glucose-6-phosphate. Glucose-6-phosphate cannot diffuse through membrane because of its negative charge. So, it is isomerised to form fructose-6-phosphate. Fructose consists of carbonyl group at carbon-2 which causes cleavage of bond between carbon-3 and carbon-4. So, option (b) is correct.
21. Presence of which one of the following in the urine indicates pregnancy in human?
(a) Oestrogen
(b) Follicle-stimulating hormone and luteinsing hormone
(c) Progesterone
(d) Human chorionic gonadotropin
Ans. (d)
Sol. The human Chorionic Gonadotropin (hCG) is made by the placenta during pregnancy. The presence of hCG in urine is check for pregnancy. So, option (d) is correct.
22. 
(a) –1
(b) 2
(c) 1
(d) –2
Ans. (b)
Sol.


23. In plants, the ovules are attached to the ovary by
(a) tube cells
(b) placenta
(c) embryo sac
(d) synergids
Ans. (b)
Sol. Placenta is the surface of carple to which ovules are attached, Placenta is commonly located submarginally in a simple pistil.

24. A stationary enemy ship is docked in the sea at a distance of 1.0 km from the coastine. A gun located at the sea level on the coastline can fire projectiles at a velocity of 120 m/s. What is the angle (in degrees) above the horizontal at which the gun must fire to hit the ship? [g = 9.8 ms/2]
(a) 21.4
(b) 42.9
(c) 47.1
(d) 23.6
Ans. (a)
Sol. Given that,

25. What is the role of bile salts in the mammalian digestive system?
(a) Bile salts emulsify fat, and thus aid in fat digestion
(b) Bile salts convert pepsinogen to pepin, and thus facilitate protein digestion
(c) Bile salts facilitate digestion of all types of macromolecules in the small intestine
(d) Bile salts are excretory products produced by the liver, and do not participate in digestion
Ans. (a)
Sol. Bile salts helps in breaking large fat molecule into small fat globules. Bile salt reduces surface tension and converts large fat molecule into smaller one, which can then be easily digested by the lipase enzyme. This process is called emulsification.
26. Which one of the following isomers is thermodynamically most stable?
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. If a bulky group is located on axial and equatorial position in an isormer. Then that isomer is the most stable. So, option (d) is correct.

27. IUPAC name of the following molecules is

(a) 3-Brome-2-isobutyl butane
(b) 4-Bromo-2-methyl-4-ethyl pentane
(c) 4-Bromo-2, 4-dimethyl hexane
(d) 3-Bromo-3, 5-dimethyl hexane
Ans. (c)
Sol.

⚫ Bromo is at 4th carbon.
⚫ Methyl group is present at 2nd and 4th carbon
⚫ It is six carbon chain, so hexane
So, 4-Bromo-2, 4 dimethyl hexane.
28. Which one of the following processes emerged earliest during the course of evolution?
(a) Thymic education
(b) Antigen presentation
(c) Antibody production
(d) Phagocytosis
Ans. (d)
Sol. Phagocytosis is the engulfment of large particles by cells. Phagocytosis plays a central role in evolution and origin of eukaryotic cell. According to endosymbiont theory of evolution, phagocytosis of archae bacteria leads to the formation of complex organelle like mitochondria and chloroplast. So, option (d) is correct.
29. The lack of linear correlation processes emerged earliest during the course of evolution?
(a) C-value paradox
(b) G-value paradox
(c) Central dogma
(d) Genetic diversity
Ans. (a)
Sol. C-value is the amount of DNA present in a haploid nucleus. It has been established that genome size does not increase uniformly with respect to complexity of species. This phenomenon is termed as–C—value paradox. So, option (a) is correct.
30. In a genetic cross between plants bearing violet flowers and green seeds (VVGG), and white flower and yellow seeds (vvgg), the following phenotypic distribution was obtained in the F2 progeny (assume both parents to be pure breeding for both the traits, and self-cross at F1 generation).
(i) 2340 plants with violet flowers and green seeds
(ii) 47 plants with violet flowers and yellow seeds
(iii) 43 plants with white flowers and green seeds
(iv) 770 plants with white flowers and yellow seeds
Which one of the following interpretations explains the above phenotypic distribution?
(a) Flower colour in this plant species is a polygenic trait
(b) Same genes control both flower and seed colours
(c) Genes for flower and seed colours are genetically interacting
(d) Genes for flower and seed colours are present on the same chromosome
Ans. (b)
Sol. In the question was have been given that 2340 plants in F2 generation are having violet flowers and green seeds which is a parental character. 47 plants with violet flower and yellow seed which is a non-parental or recombinant character. 43 plants with white flower and green seed which is also a non-parental character and 770 plants with white flower and yellow seeds which is parental character. As frequency of parental character is more than recombinant one, so we can say that both flower and seed colour genes are present on same chromosome.

Hence, option (b) is correct.
31. Which of the following is/are involved in the initiation of DNA replication?
(a) RhoA
(b) oriC
(c) sigma factor
(d) DnaA
Ans. (a, d)
Sol. OriC or origin of replicatiin is the point where replicatiion starts. Dna A is the protein which is required for the initiation of replication. RhoA is involved in terminatiion of replication whereas sigma factor is involved in transcription, not in replication.
Hence, option (b and d) are correct.
32. Which of the following is/are common to both prokaryotic and eukaryotic gene expression?
(a) Presence of the sequence TATA in the promoter
(b) Coupled transcription and translation
(c) Post-translational modification
(d) Genetic code
Ans. (a, d)
Sol. (a) TATA box is a DNA sequence found in promoter region of both prokaryotes and eukaryotes. This DNA sequence is present 30 base pair upstream of the transcription start site.
(b) In case of eukaryotes, transcription and translation occurs in nucleus and cytoplasm whereas in prokaryotes both, transcription and translation takes place in cytosol.
(c) Post-translational modification occurs in eukaryotes only
(d) Genetic code is universal for all organisms whether prokaryote or eukaryote.
33. A changed particle accelerated by a potential V moves in a circular path with a velocity v in a uniform magnetic field B that is perpendicular to the motion. Which of the following is/are correct if the value of V is increased?
(a) Time period of the motion increases
(b) Work done by the magnetic field increases
(c) Radius of the circular path increases
(d) Kinetic energy of the particle increases
Ans. (c, d)
Sol.

34. A function f : D  R is defined as f(x) =
R is defined as f(x) =  , where D
, where D  R is the domain. The domain(s) on which the function f(x) is one to one is/are
R is the domain. The domain(s) on which the function f(x) is one to one is/are
(a) Natural numbers
(b) Integers
(c) Rational numbers
(d) Irrational numbers
Ans. (a, b)
Sol.


So, f(x) is not one-one for irrational number.
So, option (a) and (b) are correct.
35. Infrated (IR) spectroscopy is used for determining certain aspects of the structure of organic compounds.
Which of the following statement(s) is/are false?
(a) Each element absorbs at a characteristic wavelength
(b) IR peack intensities are related to molecular mass
(c) Most organic functional groups absorb in a characteristic region in the IR spectrum
(d) None of these
Ans. (a, b, d)
Sol. IR spectroscopy is used to determine functional group of molecules. In this spectroscopy analysis of interaction of a molecule with infrared light is done. Analysis is done by measuring reflection, emission and absorption of infrared light.
36. Which of the following is/are about the electron carrier, ubiquinone (coenzyme Q)?
(a) Being small and hydrophobic, ubiquinone readily shuttles between protein-based electron transfer complexes within the membrane
(b) Its hydrophilic nature and high affinity for protons enable ubiquinone to transport protons readily within the intermembrane space of mitochondria
(c) Its ability to interact with Heme C to cytochromes enables electron transport in the mitochondrial member
(d) Its ability to accept two elections, one at a time, enables ubiquinone to function at the junction between a 2-electrion donor and a 1-electron acceptor
Ans. (a, c, d)
Sol. Ubiquinone is a hydrophobic moelcule present in inner membrane or hydrophobic core of mitochondrial membrane. It accepts electron one each from complex I and II and transfer it to complex III of electron transport chain. It is a small molecule and has ability to interact with hame group of cytochromes, due to which it is able to transport electron.
37. Cyclic AMP (cAMP) acts as a second messenger for which of the following primary signaling molecule(s)?
(a) Epinephrine
(b) Prostaglandins
(c) Cortisol
(d) Retinoic acid
Ans. (a, b)
Sol. Hormone which do not enter target cell, interact with specific receptors located on surface of target cell membrane and generate second messenger such as cAMP. The second messenger in turn carries out all the hormonal functions. Hormones like epinephrine and nor-epinephrine work through second messenger cAMP.
Also prostaglandins (PGs) exert their effects via binding to specific cell surface receptors and influence second messenger systems through G-proteins.
Hence, option (a) and (b) is correct.
38. Oleic acid, shown below is

(a) Insoluble in water
(b) A saturated fatty acid
(c) Soluble in acetone
(d) An unsaturated fatty acid
Ans. (a, c, d)
Sol. Oleic acid is monounsatruated fatty acid. It is an odorless, colourless oil which is insoluble in water but soluble in acetone and other organic solvents such as alcohol, benzene, chloroform, etc.
39. Which of the following molecular genetic technique(s) is/are used in forensic science/
(a) Restriction fragment length polymorphism
(b) Electrophoretic mobility shift assay
(c) DNA fingerprinting
(d) Coimmunoprecipitation
Ans. (a, c)
Sol. DNA fingerprinting is the modern genetic technique which is vastly empolyed in forensic scinece. Before DNA fingerprinting. Restriction fragment length polymorphism (RFLP) was used in forensic science.
40. Which of the following pairs is/are analogous structures?
(a) Human hands and bat wings
(b) Dolphin fippers and fish fins
(c) Butterfly wings and bat wings
(d) Bat wings and bird wings
Ans. (b, c, d)
Sol. Analogous organs are those organs which have same function, but have different basic structural design and origin. Dolphin flippers and fish fins are analogous organs, as both of them have different structure, but perform similar function of swimming. Similarly butterfly wings bat wings, and bird wings all are analogous as they have same function flying but have different structure.
41. The molar concentration of water is pure water is ...................... M (rounded off to 1 decimal)
Ans. (55.5)
Sol. Molecular weight of H2O is 18 gm
So, 18 gm = 1 mole
Let x = molar conc. of water is pure water

42. The number of triplet codon(s) for methionine is ......................
Ans. (1)
Sol. Codons are the group of three nucleotides. For methionine there is only 1 codon, i.e., AUG. So, answer is 1.
43. The maximum number of genotypes possible for gametes formed from a diploid cell of the genotype AaBBcCDd is ......................
Ans. (8)
Sol. Given genotype is AaBBcCDd
Maximum number of genotype = 2n
where n = number of heterozygotes

44. 1.45 g of sucrose (C12H22O11) is dissolved in 30.0 mL of water. Molality (rounded off to 3 decimals) of the resulting solution is ...................... m.
Ans. (0.141)
Sol.

45. When the molecular weight of human immunoglobulin light chain ios 24 kDa, the total molecular weight of human IgG ...................... kDa.
Ans. (0.141)
Sol.

Weight of light chain = 24 kD
No. of light chain = 2
Total weight = 24 × 2 = 48
Weight of heavy chain = 48 × 2 = 96
 48
48
+96
144 kD
46. The distance between the parallel lines 2x + 5y = 7 and 2x + 5y = 15 is (rounded off to 2 decimals)
Ans. (1.49)
Sol. Distance between two parallel lines in given by

47. The de Broglie wavelength of a proton moving at a speed of 1.0 m/s is ...................... Å.
[Planck's constant = 6.626 × 10–34 m2 kg/s; mp = 167 × 10–27 kg]
Ans. (3966 to 3968)
Sol. Given that, V = 1m/s

48. For a gene present on human chromosome 4, the maximum number of alleles that may be detected by sequencing the genome of 5 males and 10 femaes is ......................
Ans. (30)
Sol. Human chromosome 4 is an autosome
Total individual = 5 males + 10 females
= 15 individual
For a gene there are 2 allele
So, maximum alleles in 15 individual
= 15 × 2 = 30 alleles
49. The number of peptide bonds in a 20-residue linear peptide is ......................
Ans. (19)
Sol. No. of peptide bond = n – 1
Where n = no. of residue
No. of residue = 20
So, no. of peptide bond = 20 – 1 = 19
50. The amount of hydrogen required to reduce 30g of 2-butene is ...................... g (rounded off to 2 decimals).
Ans. (1.07)
Sol. Structure of 2-butane is

Molecular weight 2-butane is
= 4 × 12 + 8 × 1
= 48 + 8 = 56
No. of moles of butane =  = 0.557 moles
= 0.557 moles
Moles of H-required = 2 × 0.5357
= 1.0714 moles
Amount of H-required = 1.0714 × 1gm = 1.07
51. The equation  has a solution, where
has a solution, where  is a natural number. Then
is a natural number. Then  is ......................
is ......................
Ans. (1)
Sol. Given equation


52.

The number of chiral carbons is strychnine is ......................
Ans. (6)
Sol.

Chiral carbon is that carbon which is linked to four different functional group.
No. of chiral carbon in strychnine is 6.
53. In a compound microscope, the magnification power of the objective lens is 100x, and that of the eye piece (ocular lens) is 10x. The magnification power of the microscope is ...................... x.
Ans. (1000)
Sol. Magnificatiion power of microscope = Magnification power of objective lens × Magnification power of eye piece]
 M = 100 X × 10X
M = 100 X × 10X
 M = 1000X
M = 1000X
54. The number of polypeptide chains in a core nucleosome is ...................... .
Ans. (8)
Sol. Nucleosome consist of histone octamer each consist of a polypeptide chain

55. A double helical DNA moleucle is composed of 32 mol % of adenosine. The mol % of cytosine in this DNA molecule is ......................
Ans. (18)
Sol. Given, Adenosine = 32%
So, Adenine = Thymine = 32%
Total 32 + 32 = 64%
Amount of Guanine + Cytosine = 100 – 64 = 36
Also, Guanine = Cytosine
So, Cytosine =  = 18%
= 18%
56. In the circuit shown below, the power dissipated across the  resistor ...................... W.
resistor ...................... W.

Ans. (12)
Sol. Given circuit can be deduce as shown in figure below to find Req.


57. The velocity of blood in a blood vessel of 2.0 cm radius is 30cm/s. When the blood vessel bifurcates into 2 smaller vessels of radius 1.0 cm each, the velocity of blood in ech of the smaller vessels is ...................... cm/s. Assume that the vessel walls are rigid, and blood is incompressible.
Ans. (60)
Sol. Radius of blood vessel = 2 cm
Given
Velocity of blood = 30 cm/s
Radius of small vessel = 1.0 cm
No. of small vessel = 2

58. While performing a PCR, the student forgot to add one of the two primers. The number of molecules of single-stranded DNA produced after 25 PCR cycles is ......................
Ans. (25)
Sol. PCR helps in the amplification of both strands of DNA.
Two primers are added in PCR which synthesise two new strand for each DNA molecule
Since only one primer was added.
So, number of single-stranded DNA after 285 PCR cycle is 25.
59. At 25°C and pH 7.0, the concentrations of glucose 1-phosphate and glucose 6-phosphate are 2.0 mM and 35mM, respectively at equilibrium. The standard free energy change for the conversion of glucose 1-phosphate to glucose 6-phosphate is ...................... J/mol [R = 8.315 J mol–1 K–1].
Ans. (–729.94)
Sol. Since G-1-P and G-6-P are at equilibrium so,

60. In a population at Hardy-Weinberg equilibrium, for gene-X only two alleles, namely A and a, are found. If frequency of allele A is 0.2 and the frequency of allele a is 0.8, the frequency of allele a is 0.8, frequency of the heterozygote genotype Aa in that population will be ...................... (correct to 2 decimal places).
Ans. (0.32)
Sol.

